Have you ever paid $40 for a generic pill in the U.S. and then found the exact same drug for $5 in Canada or India? It’s not a scam. It’s the reality of how generic drugs work around the world. The same medicine, made by the same company, in the same factory, can cost six times more depending on where you live. And it’s not just about price - some countries barely use generics at all, while others rely on them for over 80% of prescriptions. Why does this gap exist? And what does it mean for patients, doctors, and healthcare systems?
What Are Generic Drugs, Really?
Generic drugs are copies of brand-name medications. They contain the same active ingredient, dose, and intended effect. The only differences are in inactive ingredients - like fillers or coatings - and, often, the price. Once a brand-name drug’s patent expires, other manufacturers can produce the same drug. In theory, this should drive prices down and make medicines more accessible. But in practice, the results vary wildly.
The U.S. Food and Drug Administration (FDA) requires generics to be bioequivalent, meaning they must deliver the same amount of active ingredient into the bloodstream within a narrow range - 80% to 125% - of the original drug. The European Medicines Agency (EMA) uses similar standards. But even with these rules, real-world outcomes differ. One study found that generic drugs made in India were linked to 54% more severe adverse events than identical pills made in the U.S., especially for older drugs where cost-cutting is most aggressive.
Why Do Some Countries Use Generics More Than Others?
It’s not about need - it’s about policy, culture, and money.
In the United Kingdom, 83% of prescriptions are filled with generics. In Germany, it’s 80%. The Netherlands? 70%. These countries have strong policies that encourage or even require pharmacists to substitute generics unless the doctor says otherwise. They also pay pharmacists and doctors to make the switch. In contrast, Switzerland fills only 17% of prescriptions with generics. Why? Because patients and doctors there still trust the brand name. And because the government reimburses brand-name drugs at nearly the same rate as generics - so there’s no financial incentive to switch.
Italy and Greece lag even further behind, with less than 25% generic use. In these countries, doctors often prescribe brand-name drugs out of habit or fear of liability. Patients, too, believe that a more expensive pill must be better - even when it’s not.
The U.S. is an outlier in another way: it leads the world in generic prescriptions - over 90% of all prescriptions are for generics. But here’s the twist: Americans pay more for those generics than almost anyone else. In 2022, U.S. drug prices - including generics - were 2.78 times higher than in other wealthy countries. Why? Because competition doesn’t always mean lower prices. In many cases, just one or two manufacturers produce a generic drug. When one of them shuts down production or raises prices, there’s no backup.
Who Makes the World’s Generics - and Where Are the Risks?
India produces about 20% of all generic drugs worldwide. It supplies 40% of the generics used in the U.S. alone. With over 750 FDA-approved manufacturing sites, India is the backbone of the global generic supply chain. China is catching up fast - its number of FDA-approved facilities jumped from 12 in 2010 to 187 by 2023.
But quality control isn’t consistent. The FDA conducts inspections of foreign factories, but they’re often announced in advance. That gives manufacturers time to clean up, hire extra workers, or hide problems. In the U.S., inspections are unannounced. That’s why some U.S.-made generics have fewer safety issues than their Indian-made counterparts - even if they’re chemically identical.
During the pandemic, when India temporarily stopped exporting 26 key active ingredients, shortages hit 22 countries. Antibiotics, blood pressure meds, and antifungals disappeared. Hospitals scrambled. Patients went without. It exposed how fragile the global supply chain is.

Price Differences That Make No Sense
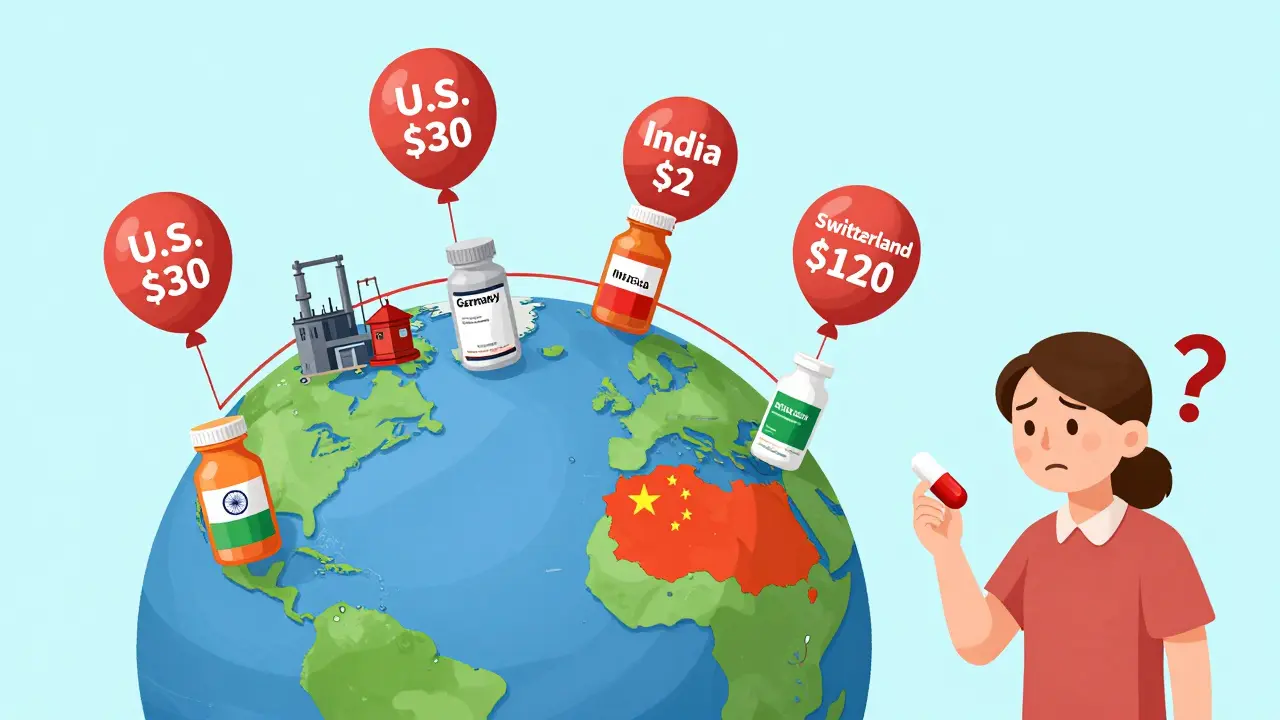
Here’s the strangest part: identical generic pills can cost 600% more in one country than another.
In Switzerland, a 30-day supply of generic metformin might cost $120. In the U.S., it’s $30. In India, it’s $2. In Germany, $8. And yet, the pill is made in the same factory, shipped in the same box, and sold under the same name.
Why? Because of how governments pay for drugs. In countries like the U.K. and Germany, the government negotiates bulk prices. In the U.S., pharmacies and insurers negotiate alone - and often lose. In Switzerland, brand-name drugs are still heavily reimbursed, so there’s no pressure to switch. In Canada, parallel importing - where pharmacies buy cheaper generics from the U.S. and resell them - is common. But it’s illegal in the U.S., even though it saves patients money.
Even within Europe, the gaps are shocking. A generic version of the blood thinner rivaroxaban costs 2.5 times more in Switzerland than in Germany. In France, it’s 40% more expensive than in the Netherlands. And yet, all these countries use the same manufacturing standards.
How Do Patients Get Caught in the Middle?
Imagine you’re on a generic thyroid medication. You’ve been stable for years. Then you travel to Canada and your pharmacist fills the prescription with a different generic - same name, same dose, but made by another company. Within days, you feel jittery, tired, or nauseous. You didn’t change your dose. You didn’t change your routine. You just changed the pill.
This happens because generics aren’t all identical. Even if they contain the same active ingredient, differences in fillers, coatings, or how the drug is absorbed can affect how it works in your body. For most people, it’s no problem. But for those with narrow therapeutic windows - like patients on thyroid, epilepsy, or blood thinner meds - even small changes can cause serious side effects.
Reddit threads are full of stories like this. One user wrote: “I switched from my U.S. generic levothyroxine to a Canadian version. My TSH levels went haywire. My doctor had to adjust my dose twice.” Another said: “I’ve had two different Indian-made metformin pills. One gave me diarrhea. The other didn’t. I never know which one I’ll get.”
Doctors don’t always know which version their patients are taking. Pharmacists don’t always know either. And patients rarely ask. The system assumes all generics are interchangeable. But they’re not.
What’s Changing - and What’s Stuck
There are signs of progress. The U.S. Inflation Reduction Act of 2022 gave the FDA more money to inspect foreign factories and speed up approval of complex generics. The European Union is pushing for a 2030 goal of 80% generic use across all member states. The WHO released a new global benchmarking tool in 2024 to help countries improve quality control.
But big barriers remain. In Europe, each country still requires separate approval for each generic drug - even after the EMA approves it. That adds 18 to 24 months to market entry. In the U.S., patent evergreening - where drugmakers file minor reformulations to delay generics - has blocked access to 1,247 new generic versions of top-selling drugs since 2015.
Meanwhile, biosimilars - generic versions of complex biologic drugs - are growing fast. They’re projected to save $45 billion by 2028. But they’re harder to make, harder to approve, and harder to swap in. They won’t solve the generic access problem - they’ll just add another layer of complexity.
What This Means for You
If you’re in the U.S., you’re getting a lot of generics - but you’re paying more than most. If you’re in Europe, your access depends on where you live. If you’re in India or Brazil, you’re likely getting affordable generics - but sometimes with unknown quality.
Here’s what you can do:
- Ask your pharmacist: “Is this the same generic I’ve been taking?” If the name or color changed, ask why.
- If you’re on a critical medication - like warfarin, levothyroxine, or epilepsy drugs - stick with one brand of generic. Don’t switch unless your doctor approves it.
- Check if your country allows parallel import. In Canada, Australia, and parts of Europe, buying generics from lower-price countries is legal and safe.
- Use tools like PharmacyChecker.com to compare prices across countries. You might save hundreds a year.
The global generic drug market is worth $450 billion. It saves lives. But it’s also broken in places. The same medicine shouldn’t cost six times more just because of where you were born. And you shouldn’t have to guess whether your pill will work - or hurt you.
Why do generic drugs cost so much more in the U.S. than in other countries?
The U.S. doesn’t negotiate drug prices like most other wealthy countries. Instead, insurers and pharmacies negotiate individually, often losing to drugmakers. Even though 90% of prescriptions are for generics, many are made by just one or two companies. When competition is low, prices stay high. Plus, the U.S. allows patent evergreening - minor changes to drugs that delay generic entry - which keeps prices up longer.
Are Indian-made generics safe?
Many Indian-made generics are safe and effective - they make up 40% of the U.S. generic market. But quality varies. A 2023 study found Indian-made generics had 54% more severe adverse events than U.S.-made ones, especially for older drugs where cost-cutting is extreme. The FDA inspects Indian factories, but inspections are often announced in advance, giving manufacturers time to hide problems. Unannounced inspections, like those done in the U.S., catch more issues.
Can I switch between different generic versions of the same drug?
For most medications, yes. But for drugs with a narrow therapeutic window - like levothyroxine, warfarin, or seizure meds - even small changes in absorption can cause side effects. If you’re on one of these, stick with the same generic brand. Ask your pharmacist to note your preferred manufacturer. If your prescription changes, talk to your doctor before taking the new version.
Why don’t all countries use generics more?
It depends on policy, culture, and money. Countries like the U.K. and Germany have laws that require or strongly encourage pharmacists to substitute generics. They also pay doctors and pharmacists to do it. In Switzerland, Italy, and parts of the U.S., doctors and patients still prefer brand names. Some believe generics are inferior. Others get paid more to prescribe brands. Without financial or policy pressure, change doesn’t happen.
What’s being done to fix global generic drug disparities?
The U.S. Inflation Reduction Act is speeding up FDA reviews and increasing inspections of foreign factories. The European Union is trying to harmonize approval rules across member states. The WHO now has a global benchmarking tool to improve quality control. But progress is slow. Regulatory fragmentation, patent tricks, and lack of price negotiation continue to block progress. Real change will need international cooperation - something that’s still rare.



Daniel Dover
14 February, 2026 . 20:19 PM
Generics work fine here in the UK. We switch automatically unless the doctor says no. Prices are low, access is high, and no one complains. Simple system, works.
Erica Banatao Darilag
15 February, 2026 . 08:07 AM
I’ve been on levothyroxine for 12 years. I switched generics once-felt like I’d been hit by a truck. My TSH spiked. Took three months to stabilize. Never again. Stick with the same brand. Even if it’s ‘just a generic.’ Your body notices the difference.
Mike Hammer
15 February, 2026 . 11:35 AM
My cousin in India gets her blood pressure meds for $1.50 a month. Same pill I pay $40 for. I asked her how she knows it’s safe. She said, ‘My aunt took it for 15 years. Still alive.’ That’s not a clinical trial, but it’s real-world data. We need transparency, not just regulation.
Kaye Alcaraz
17 February, 2026 . 10:39 AM
It’s not about where the pill is made. It’s about who’s holding the reins. In countries with strong public health systems, generics are a tool. In the U.S., they’re a commodity. And commodities get priced to maximize profit, not patient outcomes. We’re not fixing the system-we’re just complaining about the bill.
Betty Kirby
18 February, 2026 . 06:56 AM
Let’s be real-the U.S. doesn’t have a drug policy. It has a lobbying policy. The same companies that made billions on brand-name drugs now own the generics. They just repackage them and call it ‘competition.’ It’s a shell game. And we’re the suckers paying for front-row seats.
Josiah Demara
18 February, 2026 . 20:32 PM
Indian generics are not ‘unsafe.’ They’re *economically optimized*. The FDA’s unannounced inspections catch more issues because they’re not a performance. In India, the factory runs like a Bollywood set-everything looks perfect for the camera. But behind the scenes? Cost-cutting on binders, fillers, dissolution rates. It’s not fraud. It’s capitalism. And we enabled it by outsourcing everything to the lowest bidder.
Mandeep Singh
19 February, 2026 . 09:25 AM
Oh please. You Americans act like you’re the only ones who care about medicine. India produces 70% of the world’s generic vaccines and 40% of its pills. We don’t have the luxury of overpriced insurance lobbies. We make affordable drugs because our people *need* them. You want cheap medicine? Stop blaming us. Fix your broken system. We’ve been doing this for decades while you were busy patenting cough syrup.
Michael Page
20 February, 2026 . 00:39 AM
There’s an ontological paradox here. If a drug is chemically identical, and its effect is bioequivalent, then why does the patient experience diverge? The answer lies not in pharmacology, but in phenomenology. The body interprets the pill not as a molecule, but as a symbol of trust. A U.S.-made tablet carries institutional authority. An Indian one carries the weight of global inequality. The difference is not in the capsule-it’s in the context.
Esha Pathak
20 February, 2026 . 14:00 PM
India made 80% of the world’s hydroxychloroquine during the pandemic. No one died because we ran out. But in the U.S., people were hoarding it like gold because they thought it was magic. The irony? We made it cheap. You paid more for less. And now you’re surprised when your ‘generic’ doesn’t work? It’s not the pill. It’s the narrative.
Sarah Barrett
22 February, 2026 . 08:59 AM
It’s wild how we treat medicine like a luxury item. You wouldn’t accept paying six times more for the same bread just because you live in a different zip code. But for pills? We shrug. It’s not just policy failure-it’s moral failure. We’ve normalized the idea that your health should depend on your wallet. And we call that ‘freedom.’
Kapil Verma
23 February, 2026 . 07:49 AM
Stop acting like India is some third-world drug factory. We have over 750 FDA-approved facilities. We export to 150 countries. Our scientists are PhDs from IITs and AIIMS. You don’t get to call our medicines ‘questionable’ while you outsource your entire supply chain and then blame us for the cost. The problem isn’t Indian generics-it’s American greed. We make them. You overcharge. Own your mess.
Joe Grushkin
25 February, 2026 . 07:05 AM
Why do people think generics are interchangeable? They’re not. The FDA’s 80-125% bioequivalence window is a joke. That’s a 45% variance. Two pills could be 45% apart in absorption. That’s not a generic. That’s a lottery ticket. And we wonder why patients get sick? It’s not conspiracy. It’s math.
Chiruvella Pardha Krishna
25 February, 2026 . 20:23 PM
Let me tell you something. In rural India, a woman with diabetes pays $0.30 for insulin. In Chicago, it’s $200. The same vial. Same manufacturer. Same batch. The difference? In India, it’s a lifeline. In America, it’s a profit center. We don’t need new laws. We need to stop pretending that healthcare is a market. It’s a human right. And if you can’t afford it, you shouldn’t have to beg for it.