When you pick up a prescription, you might not think about whether the pill in your hand is the same as the brand-name version. But behind every generic drug is a rigorous scientific process called bioequivalence testing - and it’s the reason you can trust that your cheaper medication works just as well and is just as safe.
What Bioequivalence Really Means
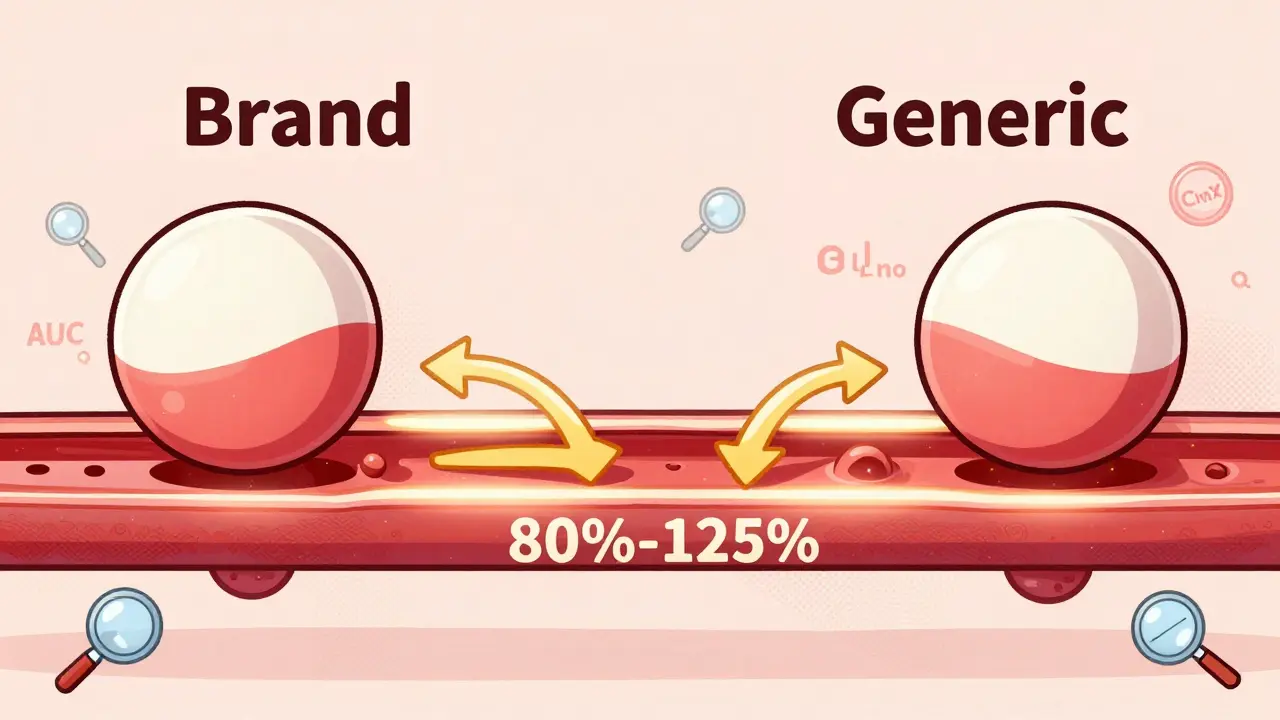
Bioequivalence isn’t just about two drugs looking the same or having the same active ingredient. It means they deliver the same amount of medicine into your bloodstream at the same speed. That’s critical. If a generic drug absorbs too slowly, it won’t work. If it absorbs too fast, it could cause side effects. The goal is to match the brand-name drug exactly - not close, not almost, but within strict scientific limits.The standard? The 90% confidence interval for the drug’s absorption - measured by AUC (total exposure) and Cmax (peak concentration) - must fall between 80% and 125% of the brand-name version. That’s not arbitrary. It’s based on decades of clinical data showing that within this range, patients experience no meaningful difference in how the drug works or how safe it is.
For most drugs, this works perfectly. But for drugs with a narrow therapeutic index - like warfarin, levothyroxine, or phenytoin - even small changes can be dangerous. That’s why regulators require tighter limits for these: sometimes as narrow as 90% to 111%. These drugs are why bioequivalence testing exists in the first place: to prevent harm.
How Bioequivalence Studies Work
These aren’t long-term clinical trials. They’re tightly controlled pharmacokinetic studies, usually done in healthy adults. Volunteers take a single dose of the generic drug and the brand-name drug, on different days, in a random order. Blood samples are taken every 15 to 30 minutes for 24 to 72 hours. Then, scientists measure exactly how much of the drug is in the blood at each time point.The study design is simple but powerful: crossover, randomized, fasting or fed state - depending on how the original drug is meant to be taken. For example, if the brand-name drug is supposed to be taken with food, the generic must be tested the same way. This isn’t just bureaucracy; it’s about real-world use. A drug absorbed differently with food could mean inconsistent control of blood pressure, seizures, or thyroid levels.
For complex drugs - like inhalers, creams, or eye drops - testing gets harder. You can’t just draw blood and measure concentration. Scientists use advanced methods: in-vitro dissolution tests, imaging, and even computer modeling. The FDA’s 2022 initiative on complex generics specifically targets these challenges, recognizing that a pill and a nasal spray behave very differently in the body.
Why This Protects Patients
You might hear stories online: “I switched to generic sertraline and felt awful.” Or “My thyroid meds didn’t work after the switch.” These are real concerns - and they’re why the FDA and EMA track every adverse event.Here’s what the data shows: from 2020 to 2023, only 0.07% of all reported adverse drug events involved generic drugs that had passed bioequivalence testing. Meanwhile, brand-name drugs accounted for 2.3%. That’s not a coincidence. It means the testing works. The system catches problems before the drug hits the market.
When a generic fails bioequivalence, it’s rejected. No exceptions. In 2023, the FDA denied approval to 12 generic versions of drugs because they didn’t meet the 80-125% range. That’s protection in action.
And the results are clear in real life. A 2022 survey by the National Community Pharmacists Association found 87% of patients reported no difference in effectiveness between generics and brand-name drugs. For levothyroxine - a drug with a narrow window - the FDA tightened standards in 2012. Since then, patient reports of instability have dropped by nearly 40%.

The Cost of Skipping Bioequivalence
Without bioequivalence testing, the entire generic drug system collapses. In 2020, generics saved the U.S. healthcare system $313 billion. That’s because they cost 80-90% less than brand-name drugs. But if those savings came with risks - if patients had to worry that their medication might not work - no one would use them.Imagine if every time you switched pharmacies, your blood pressure medication suddenly didn’t control your numbers. Or your seizure medication started causing dizziness. That’s what happens without bioequivalence. It’s not hypothetical. Before the Hatch-Waxman Act in 1984, there were no standards. Many early generics were ineffective - and sometimes dangerous.
Today, 90% of U.S. prescriptions are filled with generics. That’s only possible because patients trust them. And they trust them because they know the FDA didn’t just check the label - they checked the science.
Global Standards and Why They Matter
Bioequivalence isn’t just an American rule. It’s a global standard. The European Medicines Agency, Health Canada, the WHO, and 134 countries now require it. But not all countries do it the same way.Japan requires fasting studies even if the brand-name drug is taken with food. The U.S. tests both fasting and fed states unless safety prevents it. Brazil mandates specific medical tests for study volunteers, while other countries don’t. These differences make it harder for manufacturers to sell globally - but they also mean regulators are adapting to local needs.
The International Pharmaceutical Regulators Programme (IPRP) is working to harmonize these rules. Why? Because patients shouldn’t get different quality generics just because they live in a different country. Safety should be universal.
What About Biosimilars?
It’s important to distinguish between small-molecule generics and biosimilars. Generics are chemically identical copies of pills and injections. Biosimilars are copies of complex biologic drugs - like those used for rheumatoid arthritis or cancer. They’re made from living cells, not chemicals. That means they can’t be exact copies.For biosimilars, regulators don’t rely on bioequivalence alone. They use a “totality of evidence” approach: structural analysis, animal studies, immune response tests, and clinical trials. It’s more expensive and time-consuming. But the goal is the same: ensure the patient gets the same benefit and safety as the original.
The Future of Bioequivalence
The field is evolving. The FDA is now accepting computer models - called physiologically-based pharmacokinetic (PBPK) models - to predict how a drug will behave in the body. In 2022, they approved 17 generics using this method, up from just 3 in 2018. This could reduce the need for human studies in some cases, making development faster and cheaper.Artificial intelligence is also being explored. If a drug dissolves in a certain way in a lab test, can AI predict whether it will be bioequivalent? Early results are promising. If validated, this could cut development time from years to months.
But experts warn: technology can’t replace human testing entirely. As Dr. Lawrence Yu from the FDA noted, topical creams and inhaled drugs remain among the hardest to test. The body’s absorption is too complex to model perfectly yet.
What Patients Should Know
If you’re switching to a generic, you’re not taking a risk - you’re making a smart choice. The system is designed to protect you. If you notice a change in how you feel after switching - fatigue, dizziness, worsening symptoms - talk to your doctor or pharmacist. Don’t assume it’s the drug. It could be stress, diet, or another medication.But don’t avoid generics out of fear. The evidence is clear: bioequivalence testing works. It’s the reason you can pay $4 for a month’s supply of metformin instead of $300. It’s the reason millions with chronic conditions can afford to stay on their meds.
The next time you fill a prescription, remember: that little generic pill carries the weight of thousands of hours of science, millions of dollars in testing, and a system built to keep you safe. It’s not magic. It’s medicine - and it’s working.